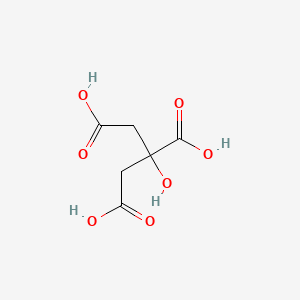
Citric Acid Ionic or Covalent
Which of the following statements about enzymes are true. On the basis of the DFT calculations table S1 P-T generally has quadruple hydrogen bonds with interaction energy of 6836 kcalmol and the averaged interaction energy 1709 kcalmol is within the range of hydrogen bond of.

Ionic Bond Vs Covalent Bond Venn Diagram Shows The Similarities And Differences Between The Chemical Bonds Click Covalent Bonding Ionic Bonding Chemical Bond
Weight concentration of a solution is expressed as ww.

. Acids are sour in taste whereas bases are bitter in taste with soapy touch. Pure acids without water are composed of covalent small molecules. A Enzymes are nonspecific b Enzymes speed up the rates of chemical reactions c Enzymes require a lot of energy to synthesize d Enzymes are not important in biological systems E Reactants in enzyme-catalyzed reactions are called substrates F Enzymes lower.
Finally the balanced equation structure and all related data for the given chemical reaction will be displayed in a new window. There are many common acid substances. Types of Compounds.
Inorganic compounds are those derived primarily from non-living sources such as rocks and minerals. How to Use Chemical Reaction Calculator. To use the chemical reaction calculator follow these steps.
Therefore we would express the concentration of this solution as sulphuric acid 50 vv. I a Ionic compounds have strong force of attraction between the oppositely charged ions eg Na and Cl so they are solids. What are acids.
Mitosis chromosome haploid diploid polyploidy prophase metaphase anaphase cytokinesis meiosis. Choose all that apply. Covalent compounds have weak force of attraction between their molecules so they are usually liquids or gases.
You have arrived exactly at the right place. The second phase occurs by the reversal of the first phase reactions and is initiated by formation of a Schiff base with the α-keto acid substrate and pyridoxamine phosphate. Based on the origin compounds can be classified into two types.
The hydrogen bond has a wide transition zone which continuously merges with the covalent bond van der Waals and ionic interaction. B Ionic compounds are soluble in water but covalent compounds are insoluble in water. As the term describes itself general engineering is the branch of science and technology that deals with many areas of science such as electrical mechanical chemical architectural civil and.
Acids are typically ionic with a positive hydrogen ion attached to a negative anion. The same type of interaction Lanthanum-modified chitosan oligosaccaharides Cos-La nanocontainers were prepared by simple ionic cross linking between oligosaccaharides and Lanthanum-citric acid complex. In the citric acid cycle ATP molecules are produced by _____.
Enter the chemical reaction into the input field Step 2. Mechanism of the first phase of transamination. These are organic compounds and inorganic compounds.
The citric acid cycle also known as the TCA cycle tricarboxylic acid cycle or the Krebs cycle is a series of chemical reactions to release stored energy through the oxidation of acetyl-CoA derived from carbohydrates fats and proteinsIn addition the cycle provides precursors of certain amino acids as well as NADH a reducing agent which are used in numerous other reactions. A few examples of inorganic compounds are common salt marble washing soda baking soda. Examples of acids are sulfuric acid hydrochloric acid acetic acid and more.
Examples of base are potassium hydroxide sodium hydroxide aluminium hydroxide and more. Lemon juice citric acid vinegar acetic acid stomach acid and soda pop carbonic acid. Like before this stands for weight per weight.
Group of answer choices. What represents a formula for a chemical compound. Click Submit to receive the results Step 3.
Chemical compounds are pure substances that are made up of two or more elements that are chemically combined in fixed mass ratios. Are you a student of engineering and looking for the largest collection of engineering quiz questions for a practice test. For example when 50ml of sulphuric acid is diluted with 50ml of water there will be 50ml of sulphuric acid in a total volume of 100ml.
When water is added. Therefore electrostatic interaction and hydrogen bonds played an important role in the loading of active herbicide in optimal-PCMs-SS carrier. The NH 2 group from the amino acid is transferred to pyridoxal phosphate with formation of the corresponding α-keto acid.
Light-dependent reactions Calvin cycle. Glycolysis Pyruvate dehydrogenase complex Citric acid cycle electron transport chain fermentation. The pH value of acid is range from 1 to 7 where the pH value of base is range from 7 to 14.


Comments
Post a Comment